This blog is suitable for both the old (R2102) and new syllabus.(Unit 1 Topic 3 Element 3)
Originally written for the old syllabus, students need to learn examples of inorganic and organic, compound and straight, liquid and granular fertilisers and give named examples for different fertilisers.
The NEW syllabus considers fertilisers in a different way. Production of synthetic fertilisers involves a lot of energy (and resulting carbon dioxide emissions) so the emphasis is on:
Improving soil health and encouraging a healthy rhizosphere so that mycorrhizae and nitrogen fixing bacteria flourish. This results in better soil based nutrition.
Using home made fertilisers such as nettle tea and comfrey tea when nutrients are recycled on site. Adding organic matter, such as garden compost, as a mulch to feed the soil.
Fertilisers are only used when nutrient deficiency symptoms are present or in containerised growing. This blog is useful as it presents a range of different fertilisers and their attributes but bear in mind that for the new qualification synthetic fertilisers should only be used when necessary: e.g. hydroponic growing.
RHS syllabus Learning outcomes (old syllabus):
Describe how plant nutrients can be provided and maintained. Identify the characteristics of organic and inorganic sources of nutrients. Define what is meant by fertilisers. State what is meant by EACH of the following terms applied to fertilisers: soluble and slow release, straight and compound, controlled release using ONE NAMED example for EACH fertiliser. State what is meant by EACH of the following terms: base dressing, top dressing, liquid feed, foliar feed, using ONE NAMED situation to illustrate the use of each. State the benefits and limitations of nutrient sources (environmental, health and safety issues, timing of application, variability of the material)
Introduction
I’m going to be honest – fertilisers bore me to death and so I don’t like teaching it. On zoom it was even more dire because usually I have a range of real fertilisers that students can look at and evaluate. So my thinking is, if I do a blog and get it off my chest, I can refer students to this blog next time it comes up and, of course, help anyone who subscribes or reads this. Also when I do a blog I usually discover new things when I’m researching or get a ha-ha moment that makes the topic more interesting, but I think the fact that it is nearly a month since the last blog says it all. I’ve been putting this one off. Anyhow I’m going to really try to make fertilisers interesting for you – I don’t know how yet, but I’m going to try, and any feedback of ideas that you use to learn or teach this topic are most welcome too. So here goes:
Any large garden centre will have a dazzling array of fertilisers lining the shelves, usually next to the chemical control section. And yes, beware, if you buy the wrong fertiliser or apply too much, it can do as much damage as any pesticide. All fertilisers are marketed to make you think your garden can not survive without them and sometimes this is true – tomatoes need tomato feed for better crops. But do you need separate fertilisers for hanging baskets of petunias and your tomatoes? – Yes and no but mainly no. Comfrey liquid feed is good for both but you may want to add a controlled release fertiliser (osmocote) to your hanging basket to save time.
We will cover the following in this blog: * Definition of fertilisers * What to consider when choosing a fertiliser * What types of nutrients are we covering? (Referring to RHS syllabus) * Why do we need to add nutrients and factors which cause nutrient deficiency? * Organic versus inorganic fertilisers. Benefits and limitations. * Fertiliser formulations and types with named examples. * Definitions for application methods and benefits and limitations of these methods. * Environmental and health and safety considerations. * QUIZ TIME * Past RHS questions and answers * Answers to QUIZ TIME
So, what are fertilisers?
Fertilisers are concentrated sources of plant nutrients that are added to growing media and taken up by roots (or sprayed on leaves as a liquid foliar feed). They are available in many forms such as granules, liquids, powders and pellets. They are used to improve growth and yield or to correct plant nutrient deficiencies. Bulky organic materials also contain a range of nutrients (garden compost, farm yard manure, municipal waste, mushroom compost) so can also be classed as fertilisers, but are not included here, in order to keep things simple. When you were studying different types of organic matter you should have evaluated nutrient and pH values of different types of bulky organic matter which also improve the structure and life in the soil as well as providing different levels of nutrients. Green manures are another way to retain and improve soil nutrient levels in the root zone. Here we will focus on the concentrated forms of fertilisers.
What to consider when choosing a fertiliser:
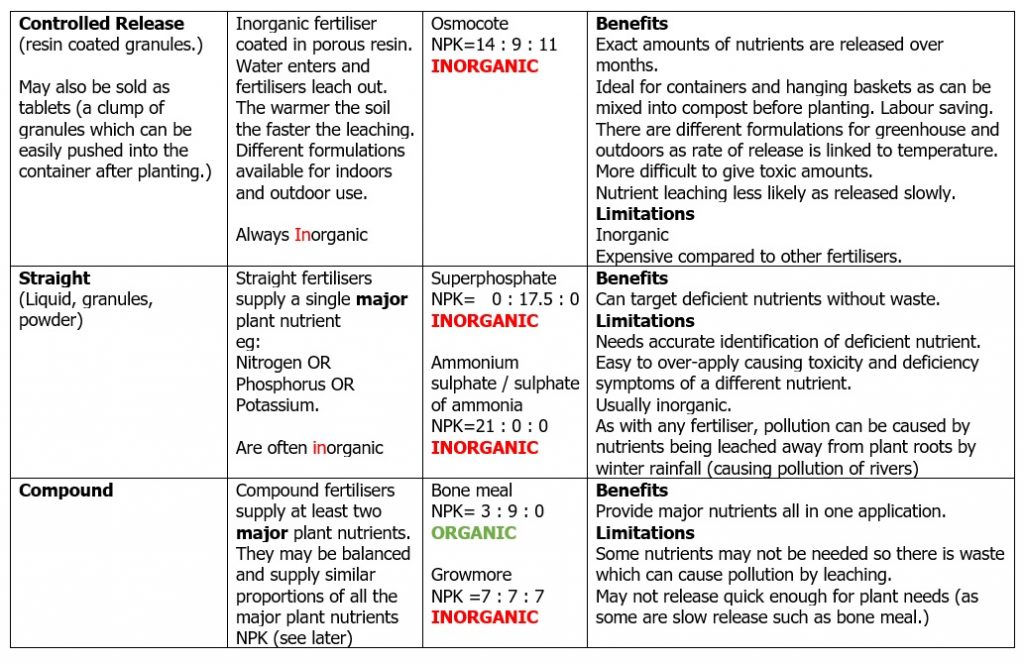
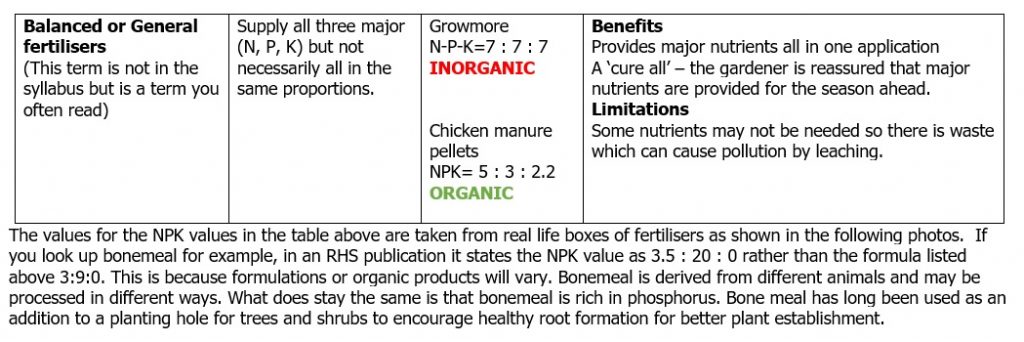
NUTRIENT CONTENT: Is it a straight fertiliser containing just one major nutrient or a compound fertiliser containing more than one major nutrient? Is the nutrient value accurate if it is a homemade liquid feed of nettles for example?
FORM: Is it a powder, granules, liquid, pellets? (Some fertilisers can either be applied as granules to the soil surface or dissolved in water to make a liquid feed.)
SPEED OF RELEASE: If a plant is suffering from a nutrient deficiency then a fast release fertiliser is preferred so the problem can be solved quickly. Organic fertilisers tend to be slower release (eg bonemeal) compared to inorganic (eg superphosphate) which release nutrients faster. (But there are always exceptions)
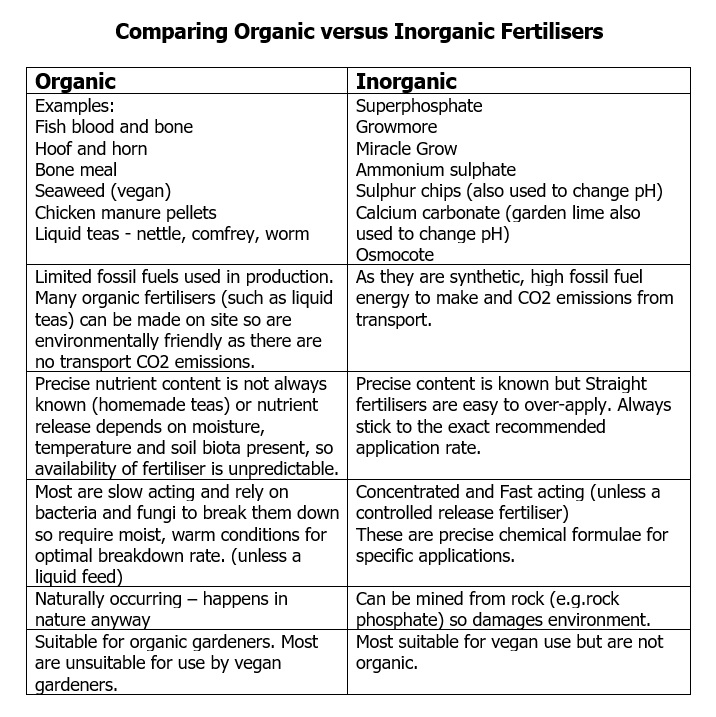
ORGANIC or INORGANIC: An organic fertiliser is derived from living organisms (plants or animals) which are decomposed. Inorganic fertilisers are mined rocks from quarries or synthetic processed chemicals. If used in community settings, the purchaser may also consider vegan-only products such as seaweed fertiliser.
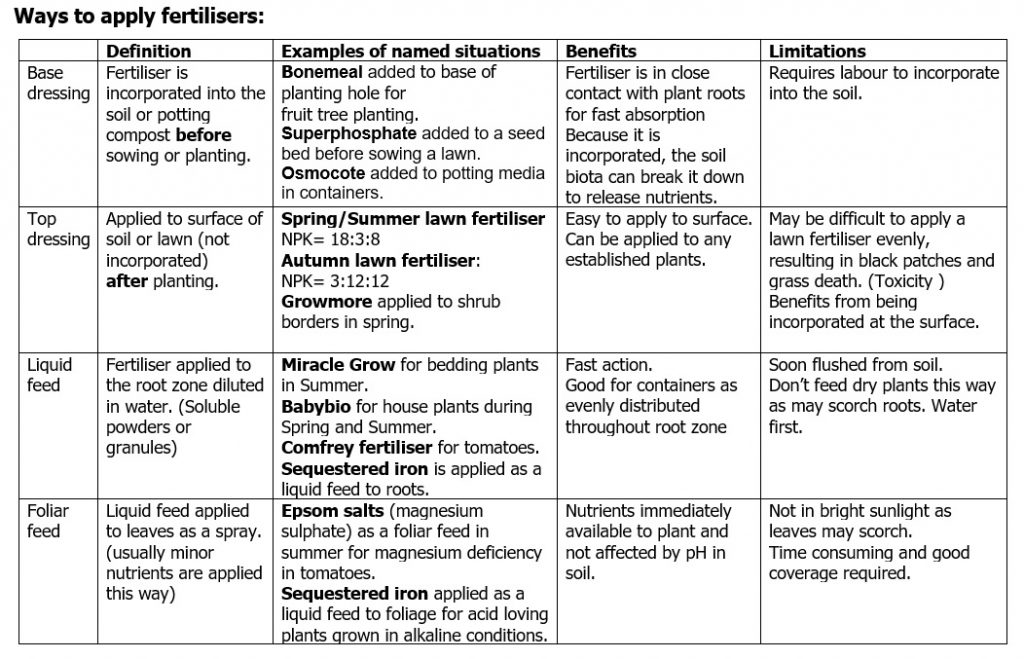
HOW IS IT APPLIED: Is the fertiliser applied as a base dressing or top dressing for example? We will define these terms later.
Before we start an overview of nutrients, which nutrients are outlined in the syllabus?
In the syllabus there are only 6 nutrients which are studied in detail; Major Nutrients (macro nutrients) required in relatively large amounts:
5 major nutrients are studied:
- Nitrogen (chemical symbol N) (required for leafy growth)
- Phosphorus (chemical symbol P) (Required for root growth)
- Potassium (Chemical symbol K) (Required for fruit and flowers) Fertiliser packaging displays a value for relative proportional content of NPK as these three nutrients (also called elements) are required in the largest amounts. To remember the three main nutrients and what type of plant growth they are needed for think: NPK = needed for shoots, roots, fruits in that order.
- Magnesium (Chemical symbol Mg)
- Calcium (Chemical symbol Ca) ( Sulphur is also a major nutrient but not studied in detail)
Minor Nutrients ( also called trace elements or micro nutrients) required in relatively small amounts - Iron (Chemical symbol Fe)
Iron is the only micronutrient studied in detail. (Copper, zinc, manganese, boron , molybdenum, chlorine, are also minor nutrients but not studied in the syllabus)
Why do we need to add fertilisers and what other factors influence nutrient status?
There are specific circumstances where fertilisers are definitely helpful:
- Containers and hanging baskets have a limited root zone and there are fewer helpful micro organisms in the growing media which help plant roots to absorb nutrients. Nutrients have to be in solution to be absorbed so containerised plants may suffer from deficiency symptoms due to irregular watering.
- Fruit and vegetables are fast growing and give bountiful harvests which requires many nutrients to make the ‘fabric’ of what we harvest and eat. Vegetables and fruits are grown in the same areas year after year so benefit from fertilisers as the soil may become exhausted, especially if no organic matter is applied to the surface which is good for soil health.
- Summer Bedding plants are fast growing and are started under protection early in the season, then planted out and repeat flower until the first frosts. They are annuals which flower over a long period of time through the Summer months and so require fertilisers(high potassium in particular for fruit and flowers) so that they repeat flower. (dead heading will also help with repeat flowering.)
- Sandy soils drain freely and nutrients are washed away or leached to the lower soil profile and cannot be reached by the roots. Leaching is when excess fertilisers are washed away by rain to other areas. However fertilisers can leach on mass to lower land areas causing pollution and damage to water habitats.
- pH is very influential in relation to availability of nutrients. A nutrient such as Iron (Fe) may be present in the soil but has formed insoluble compounds which cannot be taken up by the plant roots due to a high (alkaline) pH. A low pH also reduces the availability of the major plant nutrients , Nitrogen(N), Phosphates(P) and Potassium(K) If nutrient deficiency symptoms are present , the pH should also be checked.
- The Goldilocks Effect. The amount of fertiliser has to be the correct nutrient and the correct amount of that nutrient for that plant. If you over feed with a nutrient it will cause toxicity symptoms. For example, tomatoes often show symptoms of magnesium deficiency if over fed with a high potassium feed. Too much potassium (toxic overload) causes symptoms of magnesium deficiency. If you grow tomatoes, you often get magnesium deficiency symptoms in the lower leaves. Here’s photographic proof below. I think I am quite good at growing tomatoes but I always get magnesium deficiency symptoms. This is why it is always important to read the packaging which gives the application rate and the mantra LESS is MORE certainly applies. Better to under feed a little than apply too much.

Plants which are fast growing and in containers are the most at risk of nutrient deficiency so bedding plants, and fruit and veg in containers are the ones most at risk of nutrient deficiency and are the ones to supplement with fertilisers as required.
Symptoms of nutrient deficiency
There is another section in the syllabus where you need to know one function of each of the 6 nutrients covered, and the symptoms of deficiency. I am not going to cover this in this blog as I think it would lead to information overload. You can find this information on the RHS page here: https://www.rhs.org.uk/advice/profile?pid=456
Tables of definitions and examples

Environmental and health and safety issues
We have already covered some issues in the tables above but here is a bullet point summary overview:
- Risk of pollution: synthetic fertilisers which release nutrients quickly may leach away from the site of application and pollute water courses. Eutrophication is when excess nutrients cause structural changes to the ecosystem such as: increased production of algae and aquatic plants, depletion of fish species and general deterioration of water quality. Fertiliser application should target the specific root zone and not be generally scattered around the border so there is less wastage and potential of leaching.
- Energy used to make synthetic fertilisers is high.
- Some products are recycled waste products of the slaughter industry.
- Carbon emissions is lower for fertilisers made on site such as worm tea compared to fertilisers that are transported from processing plants. ‘Avoiding combustion of fossil fuels is therefore the most important objective in mitigating climate change.’ (RHS website)
- Mined rock compounds damage natural habitats e.g., rock phosphate and calcium compounds
- Protective personal equipment such as gloves and face mask should be worn when applying fertilisers as fine powders can be inhaled and cause skin reactions.
- When spraying foliar feeds make sure the fertiliser does not drift onto other plants.
- Correct timing for application will reduce runoff and pollution. Many are applied in Spring after the Winter rains. Spring timing maximises the opportunity for root-uptake of the added nutrients and so minimises the risk of pollution.
- Fertilisers should always remain in their original container and stored securely. The package gives information such as NPK values, optimum application rate, application method and who to contact if further product information is required.
- The inter-relationships in the soil biome are complex. Using any type of fertiliser is bound to influence this balance which may be detrimental to plant growth.
- Fertilisers should be applied on a still day so they are not blown by the wind onto other areas.
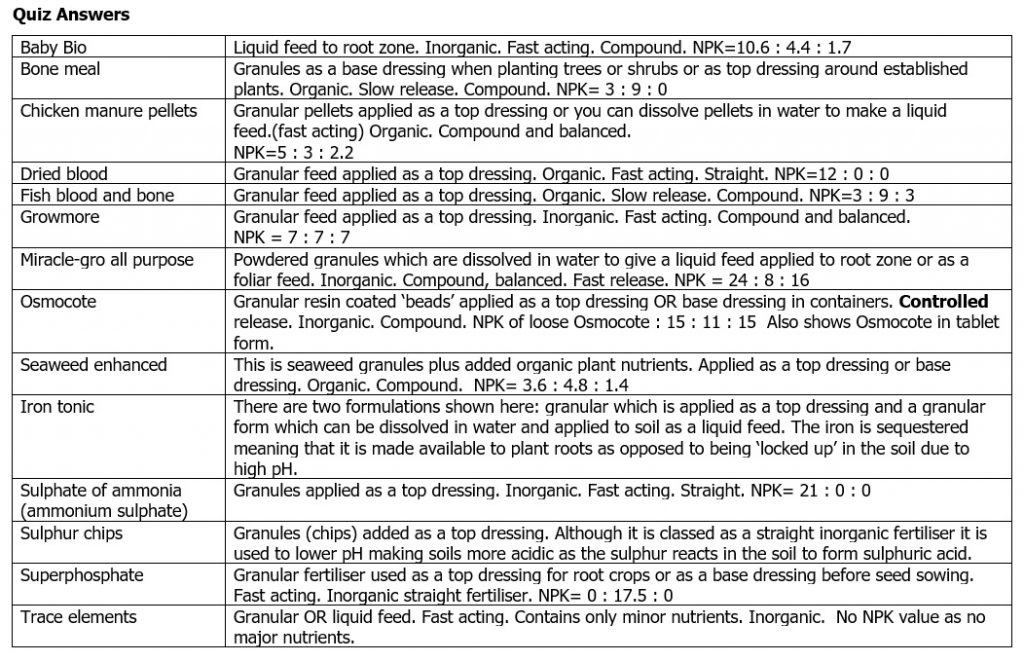
Quiz Time!
Look at each of the photos below which show a range of fertilisers. Think about the definitions above and decide the following:
- Organic or inorganic?
- Slow release, controlled release or fast release?
- Straight or compound or balanced?
- Granules, liquid, powder?
- How is it applied? Base dressing, top dressing, liquid to roots or foliar feed?
- NPK value?
Answers are at the end.














Past RHS Questions on fertilisers – the best way to revise is to do past RHS questions – see their website
Quiz Answers
THE END
Thank goodness
Glad I’ve got that topic off my chest.
Next time I teach this woopeee. All I need to say is read the blog and next week we will have a quiz.
Here’s a lovely picture of my orchid which I have been feeding with Baby Bio. It suddenly opened today much to my surprise.







